The content below is from Season 2 Episode 76 of the Who’d a Thunk It? Podcast with Zeb.
RECOMMENDATION SEGMENT
- Disney’s Atlantis: The Lost Empire
- It came out in 2001 but the animation holds up.
- Starring Michael J Fox and featuring some of the most entertaining ensemble of characters in any adventure story I’ve ever seen… I recommend you watch Atlantis: The Lost Empire.
- You’ve probably seen it before, but just re-watch it.
- My favorite characters are Cookie the cook and Vinny the explosives guy

- That scene where Cookie gives the bacon grease to Milo at the end always cracks me up.
- My dad and all his old hunting buddies always used the left over bacon grease when they cooked anything on the stove.

NOW FOR THE MAIN EVENT!
- I like things that go boom.
- I’m aware that explosives have been used for violence throughout history and are still used that way today.
- But that’s not what this episode is about. This episode is about the explosives themselves, the chemistry behind them, and how they shaped our society as a whole.

- Why do things explode?
- Let us start by explaining the chemistry behind it. Don’t worry, I’ll try to keep this brief and relatively simple. Even though I LOVE chemistry, I’m aware that most people are not big fans of the subject.
- You have the elements: Hydrogen, Helium, Carbon, Oxygen, etc.
- Atoms are the most basic unit of elements. When more than one atom binds together, scientists call this a molecule. Molecules are the building blocks of chemicals what most people call chemicals
- SIDE NOTE: Technically everything is a chemical… but usually when people refer to chemicals they mean a compound or substance that has been purified or prepared, especially artificially.
- A chemical reaction occurs when two or more chemicals, called the reactants, combine and rearrange their atoms in a way so that what comes out at the end, the product, is different than the starting materials.
- Everything wants to move toward a lower energy. Reactions are driven by energy
- Some reactions occur spontaneously like how iron reacts with oxygen to form rust, or iron oxide. The product of this reaction, iron oxide, has a lower energy than the reactants, iron and oxygen.
- But not all reactions happen spontaneously. Sometimes you gotta add energy (typically in the form of heat) to cause the reaction.
- An explosion occurs when the reaction is so favorable that there is a large release of energy. An explosion can then drive more reactions.
- The reactions keep going until there are no more molecules left to react and create a product. The energy released usually comes out as heat, which can cause fires to break out.
- SIDE NOTE: that is why you shouldn’t mix household chemicals. You can create an explosion or even poisonous gas.
- Whenever you mix ammonia and bleach it makes mustard gas. If you mix a cup of strong urine with a cup of bleach, a violent reaction will occur and you could make Chlorine gas. When cleaning the area around a toilet or when pets stains are cleaned. Both chloramine and chlorine gases are immediately irritating with a very pungent odor, causing watering of the eyes, runny nose and coughing.
- SIDE NOTE: that is why you shouldn’t mix household chemicals. You can create an explosion or even poisonous gas.
- So to sum up my chemistry lesson:
- An explosion is caused by a rapid expansion of gas from a chemical reaction. It is a violent expansion in which energy is transmitted outward as a shock wave.
- When certain elements or compounds come in to contact with each other they explode.
- Now let’s talk about some of those certain compounds:

- Gun Powder
- The term gun powder refers to a number of substances used to propel missiles out of guns and for blasting work in mines. But I will only be talking about the first of these substances to be created: Black Powder.
- Black Powder is made up of saltpetre (potassium nitrate), sulfur, and charcoal. When prepared in roughly the correct proportions (75 percent saltpetre, 15 percent charcoal, and 10 percent sulfur).
- Once you light it, black powder burns fast. What’s left is 40% gas to about 60% solid byproduct. If you light Black Powder in a confined space the gas that comes from the explosion can be used to propel things. That’s a very crude description of how a gun works.
- Black powder is sort of insensitive to shock and friction… sort of lol (meaning don’t press your luck, too much shock could still set it off). Most of the time you have to ignite (or cause a reaction) by using a an open flame or high amounts of heat.
- The gun industry has mostly switched to smokeless powder these days, but black powder is still used for ignition charges, primers, fuses, and blank-fire charges in military ammunition.
- If you switch up the ingredients just a smidge used in fireworks, time fuses, signals, squibs, and spatting charges for practice bombs.
- So Black Powder is made up of a fuel (that’s the charcoal or sugar) and an oxidizer (saltpeter or niter), and sulfur, to allow for a stable reaction. The carbon from the charcoal plus oxygen forms carbon dioxide and energy. The reaction would be slow, like a wood fire, except for the oxidizing agent. Carbon in a fire must draw oxygen from the air. Saltpeter provides extra oxygen. Potassium nitrate, sulfur, and carbon react together to form nitrogen and carbon dioxide gases and potassium sulfide. The expanding gases, nitrogen and carbon dioxide, provide the propelling action.
- Gunpowder tends to produce a lot of smoke, which can impair vision on a battlefield or reduce the visibility of fireworks. Changing the ratio of the ingredients affects the rate at which the gunpowder burns and the amount of smoke that is produced.
- History of Black Powder
- The farther back you go in history the harder it is to pin point an exact date and the culture that created Black Powder is about as old as they come. Historians believe Black Powder originated in China, where it was being used in fireworks and signals by the 10th century.
- Originally, it was made by mixing elemental sulfur, charcoal, and saltpeter (potassium nitrate). The charcoal traditionally came from the willow tree, but grapevine, hazel, elder, laurel, and pine cones have all been used. Charcoal is not the only fuel that can be used. Sugar is used instead in many pyrotechnic applications.
- When the ingredients were carefully ground together, the end result was a powder that was called “serpentine.” The ingredients tended to require remixing prior to use, so making gunpowder was very dangerous. People who made gunpowder would sometimes add water, wine, or another liquid to reduce this hazard since a single spark could result in a smoky fire. Once the serpentine was mixed with a liquid, it could be pushed through a screen to make small pellets, which were then allowed to dry.
- Between the 10th and 12th centuries, the Chinese developed the huo qiang (“fire lance”) what a cool name. It was a short-range proto-gun (that means sort-of gun). The Fire Lance channeled the explosive power of gunpowder through a cylinder—initially, a bamboo tube.
- The Chinese would light their fire lances then projectiles like arrows or bits of metal would shoot out of the other end, along with a lot of fire. I imagine they didn’t always get the proportions right and these things probably blew up in their faces a lot or the opposite of that where they’d light it and it would just fizzle a bit, a tiny puff of smoke would hiss up and no projectile would shoot out.
- By the late 13th century the Chinese were making legit guns, made of cast brass or iron. Then guns began to appear in the West by 1304, when the Arabs produced a bamboo tube reinforced with iron that used a charge of black powder to shoot an arrow. Black powder was adopted for use in firearms in Europe from the 14th century but was not employed for peaceful purposes, such as mining and road building, until the late 17th century.
- It remained a useful explosive for breaking up coal and rock deposits until the early 20th century, when it was gradually replaced by dynamite for most mining purposes.
- Before Gun Powder the world’s wars were waged up close and personal with only Bows&Arrows being the most effective long range weapon.
- Also there were no fireworks before Black Powder and we couldn’t really mine for squat.
- I know this sounds counter intuitive, but I think without Black Powder (the first explosive) the world would probably be a lot more barbaric.
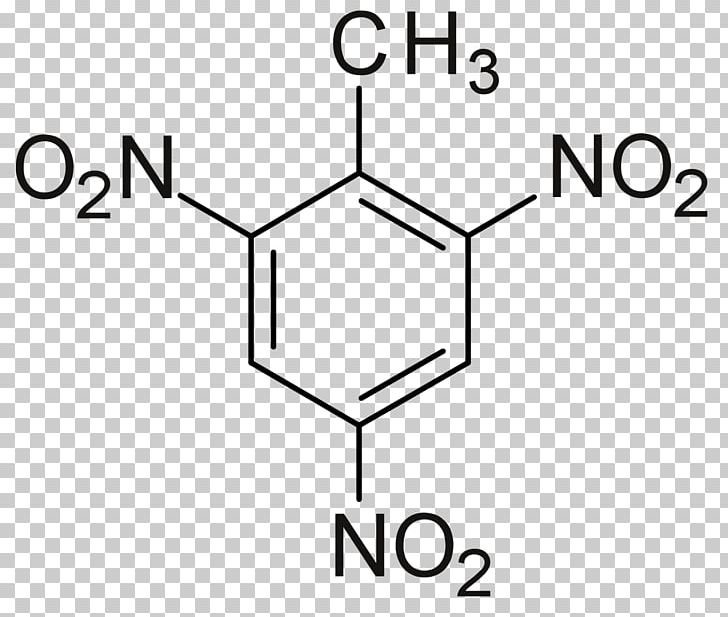
- TNT
- Can’t do an episode on explosives and not talk about TNT. It is literally the standard for measuring other explosions. When an asteroid smacks earth’s surface the news tells us how powerful the blast was by saying it how many sticks of TNT it would take to replicate the force of said asteroid. We do the same thing with other bombs.
- The “kiloton (of TNT)” is a unit of energy equal to 4.184 terajoules (4.184×1012 J)
- Trinitrotoluene more commonly known as TNT, or more specifically 2,4,6-trinitrotoluene, is a chemical compound with the formula C6H2(NO2)3CH3 (Carbon, Hydrogen, Nitrogen Dioxide, and Methyl).
- TNT is a yellow solid. It is sometimes used a s reagent in chemical synthesis, but we all know it as an explosive.
- One of the things that makes TNT so special is that it is actually hard to get TNT to explode. In order to get it to go boom you can’t just smack it against a rock or even put it in a conventional oven unless you crank the temperature up really high. TNT melts at 82° C (178° F) and does not explode below 240° C (464° F). So you can do lots with TNT before it melts your face off. That’s why militaries of the world would melt it down and pour it in to munitions’ casings all the time.
- TNT’s History
- That’s why TNT’s origins are a bit peculiar: It was first used as a dye for yellow coloring in 1863 by a German chemist named Julius Wilbrand.
- Because TNT is so insensitive to heat and shock it took 3 decades until someone realized it would make a fantastic explosive.
- In 1891 a guy named Carl Häussermann (another German Chemist) was the one who realized TNT’s potential to go *my best impression of an explosion*.
- TNT is so insensitive that it was exempted from the UK’s Explosives Act 1875. It was not considered an explosive for the purposes of manufacture and storage.
- The German armed forces adopted it as a filling for artillery shells in 1902. TNT-filled armour-piercing shells would explode after they had penetrated the armour of British capital ships, whereas the British Lyddite-filled shells tended to explode upon striking armour, thus expending much of their energy outside the ship.[8] The British started replacing Lyddite with TNT in 1907.
- TNT is poisonous,
- and if you get it on your skin it gets real itchy and irritated. TNT will also turn your skin bright yellow. During the First World War, all the men were on the fronts lines fighting in the war and the women were filling up munitions on a massive scale back home. The female munition workers who handled the TNT chemical found that their skin turned bright yellow, which resulted in their acquiring the nickname “canary girls” or simply “canaries”.
- People exposed to TNT over a prolonged period tend to experience anemia and abnormal liver functions.
- Anemia is a decrease in the total amount of red blood cells (RBCs) or hemoglobin in the blood or a lowered ability of the blood to carry oxygen. It can be rough on the heart.
- And your liver is really important.
- Blood and liver effects, spleen enlargement and other harmful effects on the immune system have also been found in animals that ingested or breathed trinitrotoluene. There is evidence that TNT adversely affects male fertility.
- TNT is listed as a possible human carcinogen, with carcinogenic effects demonstrated in animal experiments with rats, although effects upon humans so far amount to none (according to IRIS of March 15, 2000).
- If you eat TNT your pee turns red because of chemical break down… but most people who experience immediatly think it is blood in their urine so they freak out.
- Imagine that conversation with your doctor: “Doc you gotta help me. I’m pissing blood.”
- Then he comes back after running tests: “Good news and bad news imaginary patient. Good news is you are not peeing blood. Bad news is that someone has been putting TNT in to your raisin brand every morning!”
- TNT doesn’t only poison people, it pollutes its surroundings quite terribly. Residual TNT from manufacture, storage, and use can pollute water, soil, atmosphere, and biosphere.
- In Sept. of 2001, the United States Environmental Protection Agency (USEPA) declared TNT a pollutant and made the removal of TNT from military and industrial sites not just a requirement, but a priority.
- So don’t eat TNT. Don’t let you babies play with TNT. and Don’t store TNT in your basement. … it’s bad for the environment lol
- With TNT we humans were able to do so much more with explosives. I mean you can pour the stuff in to bottles… there are so many more uses for TNT than other explosives.
- Can’t do an episode on explosives and not talk about TNT. It is literally the standard for measuring other explosions. When an asteroid smacks earth’s surface the news tells us how powerful the blast was by saying it how many sticks of TNT it would take to replicate the force of said asteroid. We do the same thing with other bombs.

- Nitroglycerin
- Nitroglycerin, also called glyceryl trinitrate, a powerful explosive and an important ingredient of most forms of dynamite. It is one of the most easily ignitable explosives on my list for this episode.
- It is also used with nitrocellulose in some propellants, especially for rockets, missiles, and the race cars that used to be on the Fast and Furious movies back when they were actually about racing.
- Nitroglycerin is also used as a vasodilator in the easing of cardiac pain.
- Lots of people think TNT and Dynamite are the same thing… they are NOT.
- Pure nitroglycerin is a colourless, oily, somewhat toxic liquid having a sweet, burning taste.
- When I read that sentence doing my research I burst out laughing. I pictured a scientist in a lab coat with this stuff in a petri dish. He looks at it – takes some notes. Smells it – takes some notes. Then he tastes it and is like oh that’s not bad…. then his head explodes. THIS IS NITROGLYCERIN!!! WHY WOULD ANYONE TASTE IT?!
- It was first prepared in 1846 by the Italian chemist Ascanio Sobrero by adding glycerol to a mixture of concentrated nitric and sulfuric acids.
- The find caused a sensation because nitroglycerin’s explosive power was far beyond that of gunpowder. Ascanio thought 19th century Italy was going to have lazer guns in no time! The trouble was, nitroglycerin was highly unstable. It caused grisly explosions, including one in San Francisco that leveled a building and killed 15 people.
- Nitroglycerin, with the molecular formula C3H5(ONO2)3, has a high nitrogen content (18.5 percent) and contains sufficient oxygen atoms to oxidize the carbon and hydrogen atoms while nitrogen is being liberated, so that it is one of the most powerful explosives known. Detonation of nitroglycerin generates gases that would occupy more than 1,200 times the original volume at ordinary room temperature and pressure; moreover, the heat liberated raises the temperature to about 5,000 °C (9,000 °F). The overall effect is the instantaneous development of a pressure of 20,000 atmospheres; the resulting detonation wave moves at approximately 7,700 metres per second (more than 17,000 miles per hour). Nitroglycerin is extremely sensitive to shock and to rapid heating; it begins to decompose at 50–60 °C (122–140 °F) and explodes at 218 °C (424 °F).
- A serious problem in the use of nitroglycerin results from its high freezing point (13 °C [55 °F]) and the fact that the solid is even more shock-sensitive than the liquid. So at room temperature Nitro is super really sensitive to shock ( if you are handling it you have to do that awkward shuffle that you do when you pour a drink too full to the brim). If Nitro is in Vegas weather at like 120 degrees F it starts to decompose and become even more unstable. If Nitro is in nice fall weather at like mid 50 degrees F it freezes and when it is a solid it is even more irritable… This is one angry explosive.
- The safe use of nitroglycerin as a blasting explosive became possible after the Swedish chemist Alfred B. Nobel developed dynamite in the 1860s by combining liquid nitroglycerin with an inert porous material such as charcoal or diatomaceous earth. Nitroglycerin plasticizes collodion (a form of nitrocellulose) to form blasting gelatin, a very powerful explosive. The Nobel prize is named after Alfred B Nobel…. yeah the internationally recognized prize for peace is named after a guy who made one fo the deadliest explosive a WHOLE lot easier for the world militaries to use.
- At about the same time Nobel was perfecting dynamite, scientists in Britain were using a molecule called amyl nitrite to treat angina, an excruciating chest pain connected with inadequate flow of blood and oxygen to the heart.
- Noting similarities between amyl nitrite and nitroglycerin, London physician William Murrell became the first to recommend nitroglycerin as a treatment for angina in 1879. He did so after carrying out several studies with nitroglycerin (on himself as well as on other people).
- The World Health Organization considers nitroglycerin one of its essential medicines for a basic health system. Even Alfred Nobel got a prescription for nitroglycerin from his doctor. Nobel declined the medication, and wrote about it in a letter:
- My heart trouble will keep me here in Paris for another few days at least, until my doctors are in complete agreement about my immediate treatment. Isn’t it the irony of fate that I have been prescribed [nitroglycerin], to be taken internally! They call it Trinitrin, so as not to scare the chemist and the public.
- Another fun use of Nitro is that is can help keep your pecker pecking lol. The same blood-flow-stimulating properties that make nitroglycerin such a useful medication for relieving chest pain also may “enable a longer lasting sexual experience,” according to UK firm Futura Medical.
- The company’s nitroglycerin gel, with the brand name of Zanifil, goes inside a latex condom. It is designed to stimulate blood flow to sustain men who report having trouble keeping erections with a condom. (The goal is to encourage safer sex by convincing those same men to stick with condoms.)
- As an explosive Nitro is testy as heck! But when combined with Nobel’s porous material it is super handy! Plus the compound is good for your heart and can keep your willy hard!
- Nitroglycerin, also called glyceryl trinitrate, a powerful explosive and an important ingredient of most forms of dynamite. It is one of the most easily ignitable explosives on my list for this episode.
- There are a lot of explosives out there. If I covered them all this episode would be long as poop. Instead I think I discovered a pattern.
- It seems all explosives aren’t just used for destruction and violence. All of them seem to have a beautiful Yin and Yang to them.
- For example: Without doing any research I know nuclear power can be used as THE greatest destructive force wielded by man or it can be a virtually infinite source of electric power to help improve the lives of everyone.
- I’ve said it before and I’ll say it again. Tools aren’t evil. It is the ones who wield them.
- I swear I wasn’t trying to make a political statement with this episode. Just wanted to learn about explosives.
- I think I might be a bit of a pyromaniac, but like the explosive version of that. Kind of like the explosives expert archetype from half of every heist/adventure movie. Like Vincenzo “Vinny” Santorini from that Disney movie Atlantis.
- They fascinate me.


CREDIT
- https://www.columbiatribune.com/article/20130508/lifestyle/305089870
- https://www.britannica.com/technology/black-powder
- https://www.thoughtco.com/gunpowder-facts-and-history-607754
- https://en.wikipedia.org/wiki/TNT
- https://www.britannica.com/science/trinitrotoluene
- https://www.forbes.com/sites/carmendrahl/2016/10/01/three-things-to-know-about-dynamite-the-reason-we-have-nobel-prizes/?sh=3afc086d5cdd

